Nuclear medicine
Molecular Radiotherapy Dosimetry
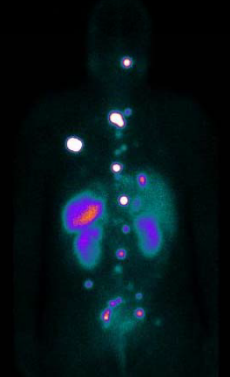
Molecular Radiotherapy (MRT) is a cancer therapy technique in which radioactive pharmaceuticals are administered inside the body to deliver a lethal radiation dose to malignant cells whilst sparing surrounding healthy tissue. There is now an extensive clinical portfolio of MRT procedures which has been significantly enhanced with the introduction of new radiopharmaceuticals. MRT treatment is generally minimally invasive, incurs few side effects and can achieve impressive results.
When delivering MRT, the radiation doses to the tumour and normal tissue should be accurately determined for each patient using nuclear-medicine imaging - an essential component in the diagnosis, staging and management of many oncological conditions. Due to the inherent complexity of measuring radiation doses from in vivo decays current MRT dose calculations are still non-optimised generic averages based on theoretical organ dimensions without regard for individual patient size and weight. Ultimately because of the uncertainty in determining doses for radiation sensitive organs, a significant proportion of patients do not receive critical tumour doses and therefore, the greatest therapeutic benefit.
Our collaboration between the Manchester nuclear-physics group and the nuclear-medicine group at The Christie NHS Foundation Trust has established a fruitful interdisciplinary research program which seeks to address the complex and interconnected problem of providing accurate patient specific dosimetry for MRT. Our research focuses on two key aspects of MRT dosimetry,
1. The application of Monte Carlo simulation techniques to improve activity quantification and dosimetry calcual- tions for a variety of MRT treatments.
2. The validation of dosimetry calculations using realistic patient analogues.
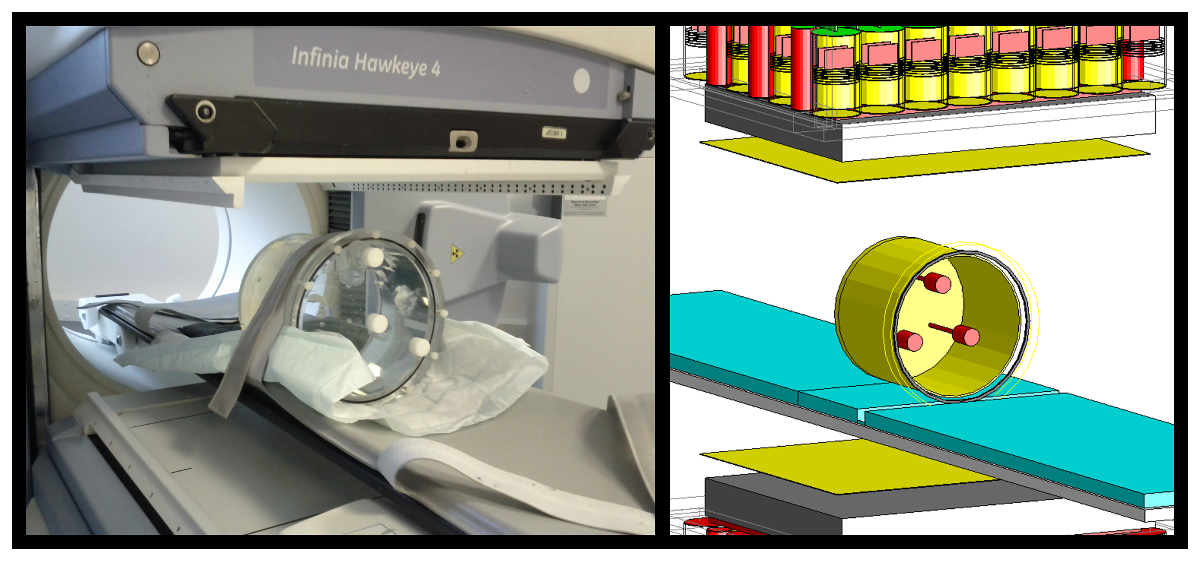
By using a detailed Monte Carlo simulation of a GE Infinia Hawkeye 4 SPECT/CT camera (as used at The Christie) we can fully track gamma rays and beta particles interactions from the initial decay within a patient body until detection in the SPECT camera. Our simulation has been used to model SPECT scan acquisitions of simple water filled phantoms (approximating the make up of the human body), more complex anthropomorphic human shaped phantoms and CT images of patient therapies. By characterising the scattering of gamma rays in the SPECT camera system we have been able to significantly improve the image quality and accuracy of activity quantification.
Our research group is also focused on the development of 3D printing techniques to produce organ models to facilitate the validation of MRT dosimetry for realistic patient morphologies. These models allow many of the underlying systemic sources of error in MRT dosimetry calculations to addressed and provide a major step towards providing individualised patient-specific treatment plans for MRT.
The knowledge exchange and output from this project has had direct impact at The Christie. During our IPS grant we have extended our collaboration with a commercial partner HERMES Medical Solutions Ltd. This leading supplier of cross platform nuclear medicine software provides a pathway to translate our research into a commercial product. Our research is currently funded by a STFC IPS grant, “Improved Organ Dose Determination & Imaging in 177Lu Targeted Radionuclide Therapy – towards a clinical solution”. Our group currently has a two PhD students (one funded by an STFC CASE award) working on projects related to this research.
Research Staff
- Dr D. M. Cullen
- Dr A. Robinson
